Diabeloop introduces an automated management solution for highly unstable diabetes
Date
Share
DBL-hu, a medical device dedicated to the autonomous management of highly unstable diabetes1, obtained its CE mark less than a year after the launch of its pivotal clinical trial began.
________________________________________
Diabeloop announced today that it has obtained CE marking for its innovative and adjustable technological solution: DBL-hu. DBL-hu improves glycemic control and satisfaction for people living with a form of diabetes that is very complex to manage. Diabeloop expands its position in therapeutic artificial intelligence, following CE marking of DBLG1 in 2018.
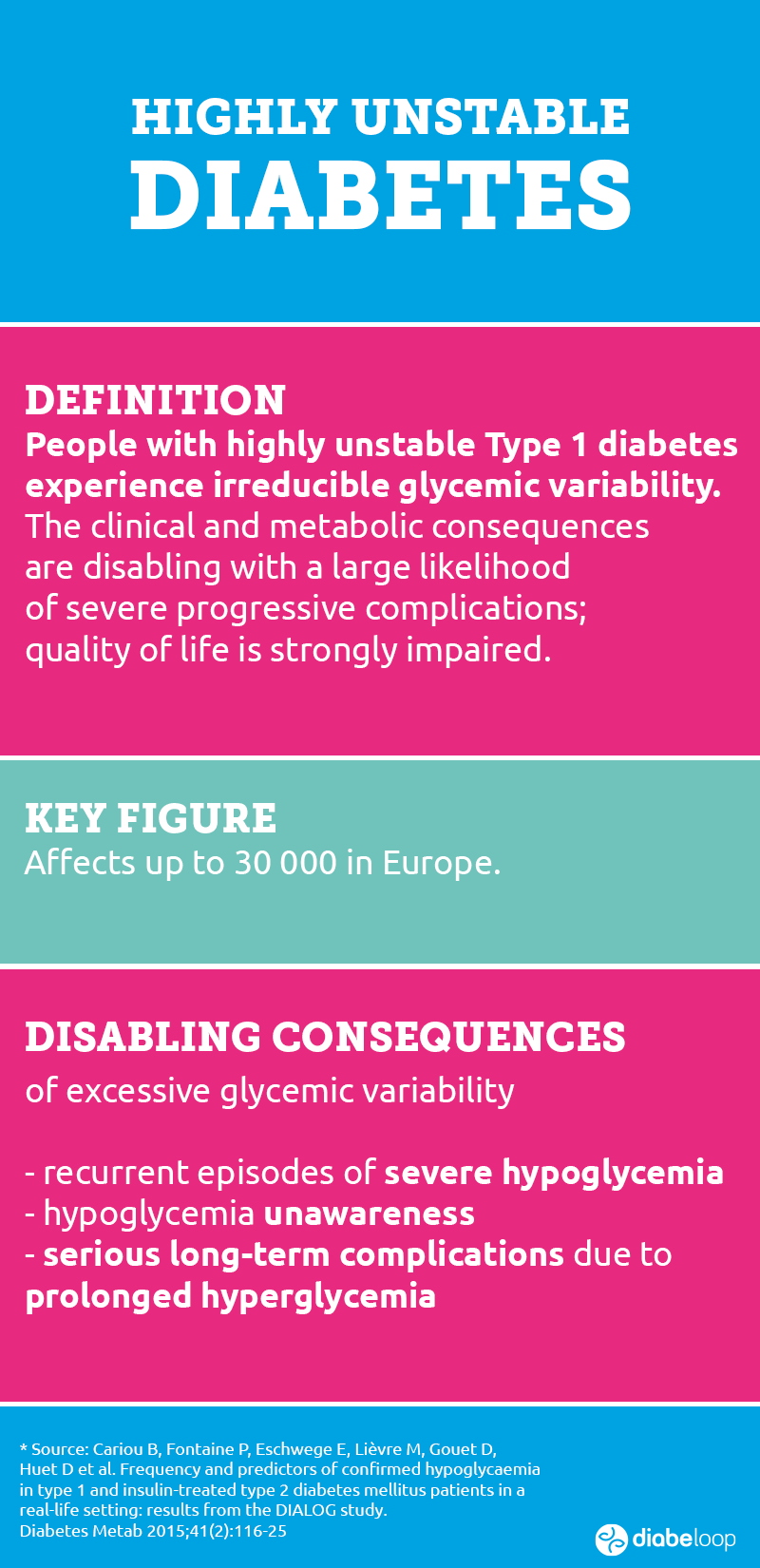
Highly unstable diabetes: people in therapeutic distress
_______________________________________________________________________
While innovative treatments for the 250,000 people living with Type 1 diabetes in France, 2.0 million in Europe and 1.5 million in the USA are helping to improve their daily lives, highly unstable diabetes – also called labile diabetes – remains complex to manage and balance with the solutions currently available on the market.
Hélène Lefebvre, Project Leader for DBL-hu at Diabeloop, explains the genesis of this project: “A very small proportion of people with Type 1 diabetes have what is called highly unstable diabetes. There aren’t two identical reasons in two different people; the researchers do not understand, to date, what creates this physiological upheaval and what leads these patients to have sudden variations between very high and very low blood sugar levels, even more so than other people with Type 1 diabetes. Several diabetologists have told us that DBLG1 System from Diabeloop is working well and that something should be done for these patients. So we started the DBL-hu project keeping in mind that we were going to have to adapt the algorithm for these people with very complex physiologies to stabilize”.
DBL-hu: an adapted and individualized response to a true need
______________________________________________________________________________
Today, few alternatives are available to people living with highly unstable Type 1 diabetes. An improved implantable insulin pump will not be available in France for a few years. Surgical options exist, with pancreas transplantation and islet transplantation. However, these procedures do not offer an absolute guarantee of success and require heavy immunosuppressive treatment. Individuals who participated in the DBL-hu clinical study had a contraindication to islet transplantation.
The option offered by Diabeloop with its closed-loop system – DBL-hu – allows patients and their physicians to consider a less invasive and flexible solution. With this device, they can enjoy a more stable daily life, a lighter mental load, reduced risk of hypoglycemic episodes and much better diabetes control.
Erik Huneker, co-founder and CEO of Diabeloop says:
“With Professor Pierre-Yves Benhamou, we discussed this problem and quickly decided to launch this clinical trial on a few patients, in an attempt to improve their lives. By customizing the device according to the patients, we quickly obtained very positive results, i.e. a real “life solution”. A diabetologist who had heard of DBL-hu explained to us that he had a patient for whom nothing worked. He wanted to offer her this treatment, except that we could not include her in the clinical study, we did not have the CE mark… We then asked the ANSM (French Health Regulation Authority) for authorization to equip this patient on a compassionate basis, an exceptional exemption for a medical device. In view of our excellent results, we obtained this authorization to include the patient in a regulatory framework. At the same time, we submitted our CE marking file which was going to solve the problem of these patients with highly unstable T1D”.
Extremely promising clinical trial results
____________________________________________________
Diabeloop, which has developed a medical device for people living with Type 1 diabetes, took an interest in these patients in therapeutic distress and quickly launched a clinical trial that resulted in CE marking in less than a year.
During the clinical trial, 7 patients were randomized. The use of the DBL-hu system was associated with a gain in time spent in the ideal glycemic range (70-180 mg/dl) of 29.8% in absolute value (73.3% of the time versus 43.5%), and also improved glucose variability, satisfaction score and perceived frequency of hypoglycemia.
These results demonstrate a significant medical breakthrough in highly unstable Type 1 diabetes.
Professor Pierre-Yves Benhamou shed light on the success of the clinical study that led DBL-hu to CE marking:
“By analyzing the very good results of the closed-loop on T1D patients, we launched this clinical trial by adjusting the closed-loop with settings that take into account these profiles of patients with more unstable metabolism. These patients have abrupt variations, they can go up to 300mg/dl in a few seconds and suddenly go down to 50mg/dl. We included 7 patients, theoretical islet transplant candidates, in this study. Engineers added nearly 3 times more settings to DBLG1 to adapt it to highly unstable diabetes. Thanks to these settings, blood glucose levels were much more stable, with some patients almost doubling time in range, from 35% / 40% to more than 70%. Patients are much more confident thanks to the DBL-hu system, managed with their diabetologist, the settings are done quickly and the results are effective from the very first days, blood glucose levels are much more stable. We have confirmed this during the 4 sequences performed on these targets, in random order: with the DBL-hu system or a standard treatment and the order of passage drawn at random; switching from the open-loop to the closed-loop improved the results and conversely, switching from the closed-loop to the open-loop deteriorated them”.
Patient testimonials during the clinical trial:
“To give you a closed-loop evaluation of the past month, less subjective than my patient opinion, here is the evolution of my glycated hemoglobin level: before the study 8.1% in April 2019 then 8.2% in August 2019 and …. 7.28% this Saturday! I am so happy!”
“I have noticed since the beginning of the closed-loop that my quality of life has increased dramatically, as the system clearly allows the management of hypo and hyperglycemia. The result is that I no longer have hypo during the night, so I am really less tired and the management of hyper has the same effect.
Following the rapid CE marking of DBL-hu, the next step is to obtain reimbursement that would allow the device to be offered to healthcare teams and people living with highly unstable diabetes.
For more information on Diabeloop: https://www.diabeloop.com/
_____________________
About Diabeloop
Diabeloop’s mission: to relieve people living with Type 1 diabetes from dozens of daily therapeutic decisions and reduce their heavy mental burden. Initially conceived from a medical research project, Diabeloop was created in 2015 by Dr. Guillaume Charpentier, now Chief Medical Officer, and Erik Huneker who have co-managed the company with Marc Julien since 2016. This complementary management team works with experienced partners, CEA-Leti (a research laboratory) and CERITD (a French research team of diabetologists).
In November 2018, the DBLG1 System, Diabeloop’s first medical device for automated diabetes management, obtained CE marking. A second round of financing of 31 million euros concluded in November 2019 to speed up the international commercial rollout of the DBLG1 iController and support an ambitious R&D program.
Today, Diabeloop gathers the personality, the passion and the skills of 80 talented individuals who work hard to improve the quality of life for every person living with Type 1 diabetes.
——————
1. Diabetes Obesity and Metabolism, October 2020: Benhamou PY, Lablanche S, Vambergue A, Doron M, Franc S, Charpentier G. Highly unstable type 1 diabetes eligible for islet transplantation can be managed with closed-loop insulin delivery. A series of N-of-1 randomised controlled trials.